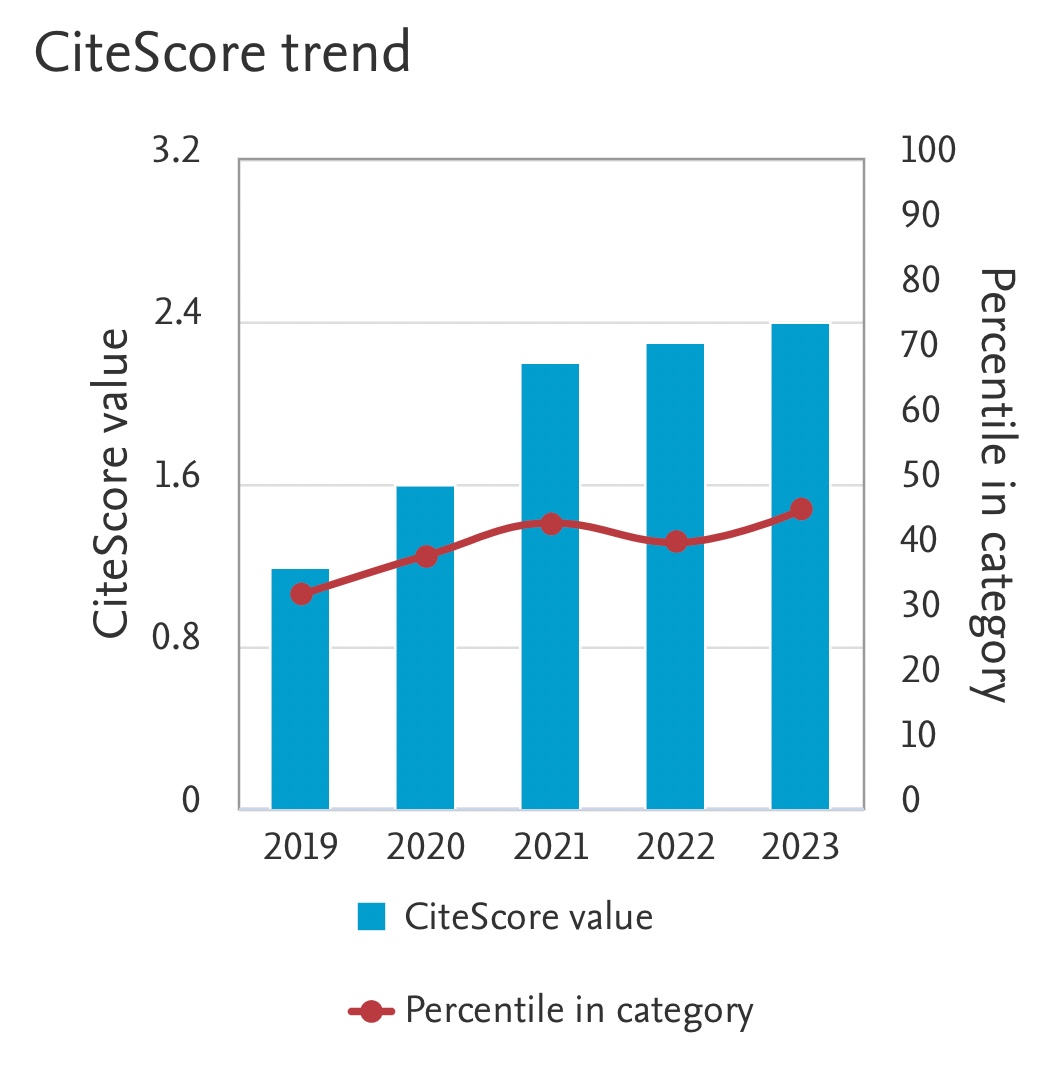
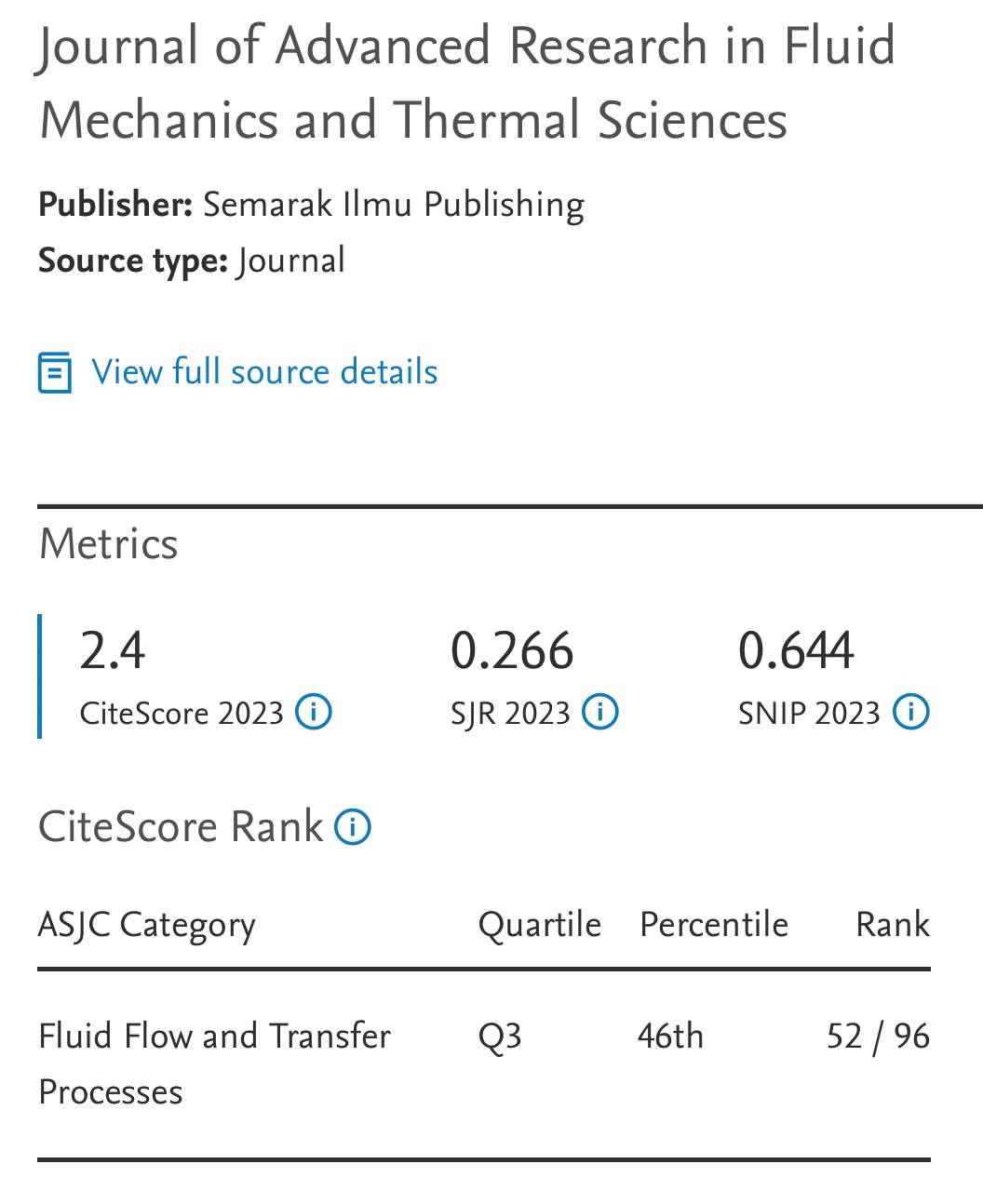
Solubility of CO2 in Piperazine (PZ) Activated Aqueous N-Methyldiethanolamine (MDEA) Solvent under High Pressure
Keywords:
Absorption, carbon dioxide solubility , N-methyldiethanolamine , piperazineAbstract
In this research work, piperazine (PZ) was used to activate the performance of aqueous N-methyldiethanolamine (MDEA) solvent for the capturing of CO2. The solubility experiments were conducted at a temperature and pressure ranges of 303.15 to 333.15 K, and 2 to 50 bar, respectively. The solubility of CO2 was investigated in terms of CO2 loading capacity as the ratio between the quantities of moles of CO2 absorbed to the total amount of moles of solvent used. It was observed that CO2 loading has increased with increasing the partial pressure of CO2 while increasing the temperature has a negative effect as the loading capacity has decreased with increase in system’s temperature. The CO2 loading capacity of the solvent investigated in this research work has demonstrated better performance as compared to conventionally used alkanolamines solvents.
Downloads