Development of Tertiary Structure of Laccase from Geobacillus uzenensis using Homology Modelling
DOI:
https://doi.org/10.37934/araset.62.1.151157Keywords:
Laccase, homology modelling, protein structure predictionAbstract
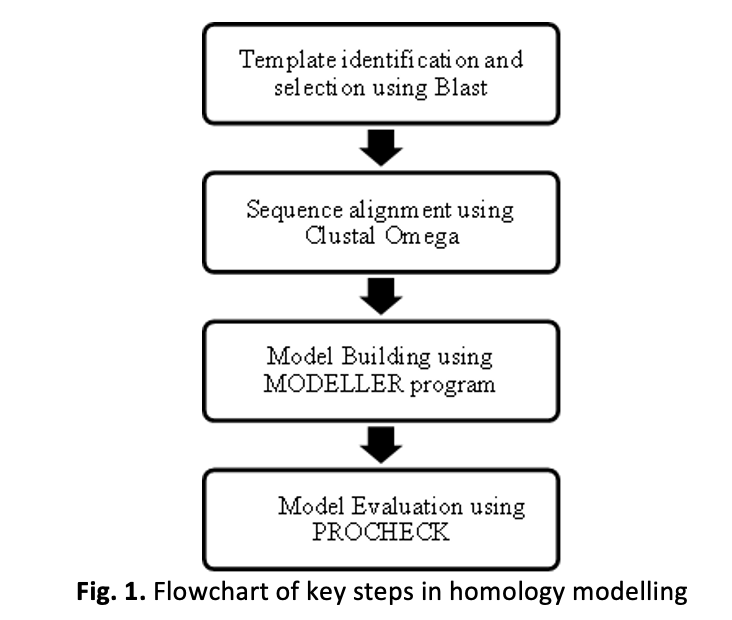
Laccases are a versatile enzyme with immense potential in various biotechnological applications. In this study, the protein structure prediction of laccase from Geobacillus uzenensis, a bacteria known for its robust enzymatic activity is focused. The protein structural elucidation of this laccase is crucial to understand its substrate binding and overall functionality. Protein structure can be determined by computational prediction, which is commonly known as homology modelling. The three-dimensional (3D) model was built based on the available crystal structures of related laccase enzymes and aligned with the primary sequence of the target protein. The resulting model exhibited a high degree of structural similarity and conservation of key catalytic residues, supporting its reliability for further analyses. Firstly, the suitable template was identified with 85.61% of sequence identity determined by sequence alignment. Then, MODELLER program was used to predict the model using the method of satisfaction of spatial restraints. The model was then analyzed for its quality by computational analysis tools such as Ramachandran's Plot. Finally, the binding site of the protein was identified using the GRaSP computational tool. These findings provide significant insight on comprehensive analysis of the laccase from G. uzenensis, by providing a structural framework to unravel its functional properties and substrate specificity.