Hydrothermal Catalytic Transformations of Polymeric Wastes over Zeolite Catalysts Modified with Molybdenum and Tungsten
DOI:
https://doi.org/10.37934/araset.57.1.117133Keywords:
Natural zeolite, Composite catalyst, Polymer waste, Hydrogenation thermocatalytic processing, AdsorptionAbstract
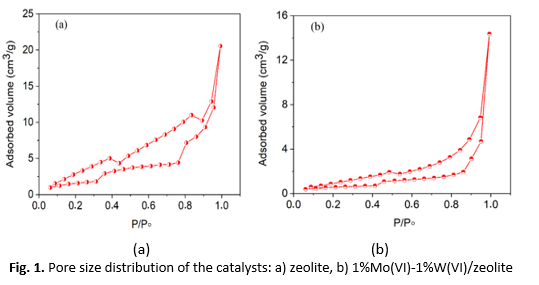
The article is devoted to the study of the adsorption properties and morphology of new composite catalysts based on acid-activated zeolite heulandite-clinoptilolite of the Kazakhstan Taizhuzgen deposit modified with molybdenum salts (NH4)MoO·4H2O and tungsten (NH4)5H5[H2(WO4)6]·H2O, for the process of thermocatalytic hydrogenation of plastic waste. The study of the developed catalyst by the method of adsorption nitrogen porometry demonstrated the presence of typical type IV isotherms, which indicated the formation of a porous adsorbent by multilayer nitrogen adsorption in the mesopores of the catalyst. The adsorption capacity of the structure formed by the catalyst based on zeolite modified with molybdenum and tungsten, due to the presence of predominantly mesoporous cavities, is able to selectively influence the process of hydrothermocatalytic processing of waste polymers based on polyethylene and polypropylene. The pore size distribution indicates the presence of mesopores and an insignificant number of micropores at low pressure. The catalyst samples were tested in the thermocatalytic hydrogenation reaction of polymer waste. Significant gas formation and predominant content of alkanes, isoalkanes, alkenes and aromatic hydrocarbons in liquid products are shown. It is obviously that the main contribution to the transformations made by mesoporous inclusions in the cavities of the zeolite under study formed during their treatment with molybdenum and tungsten salts.