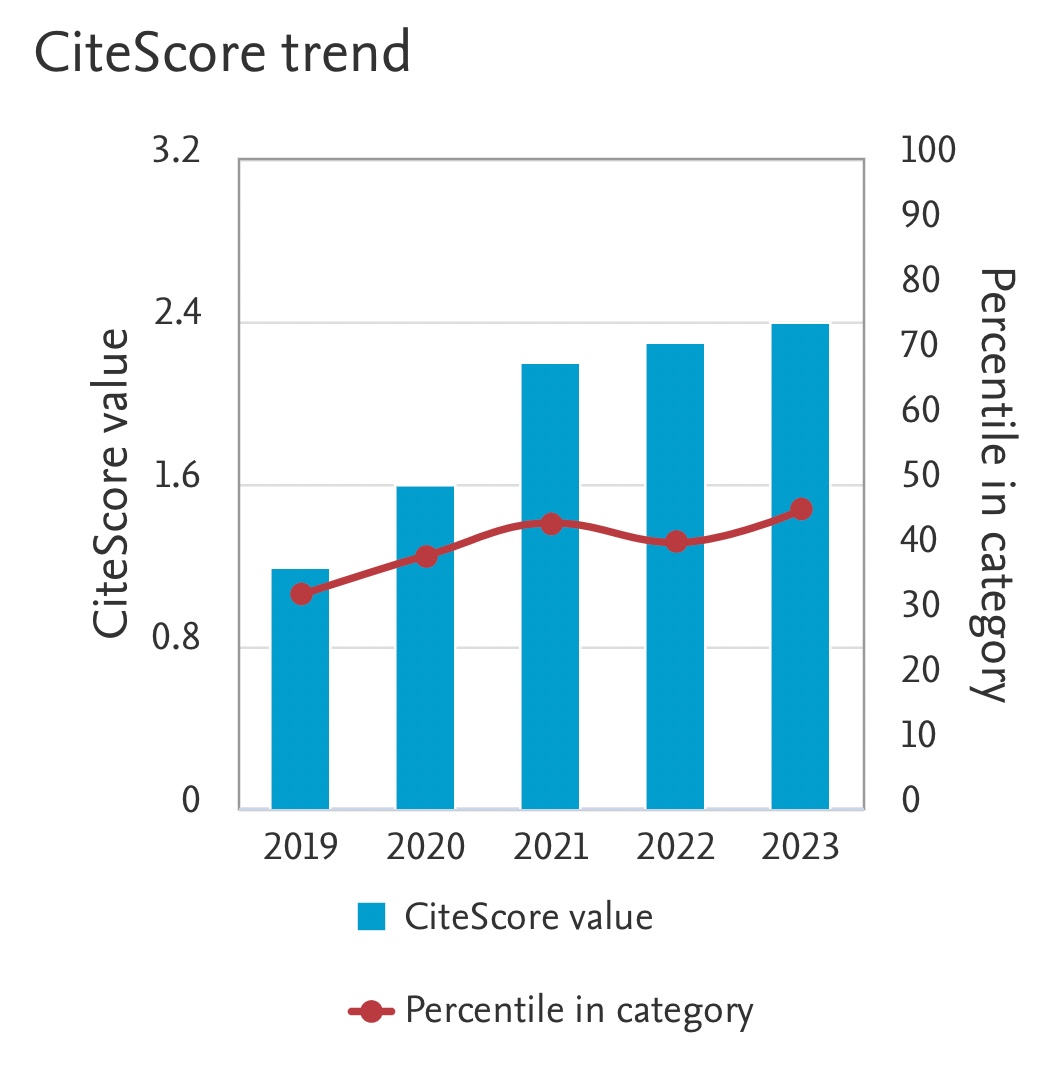
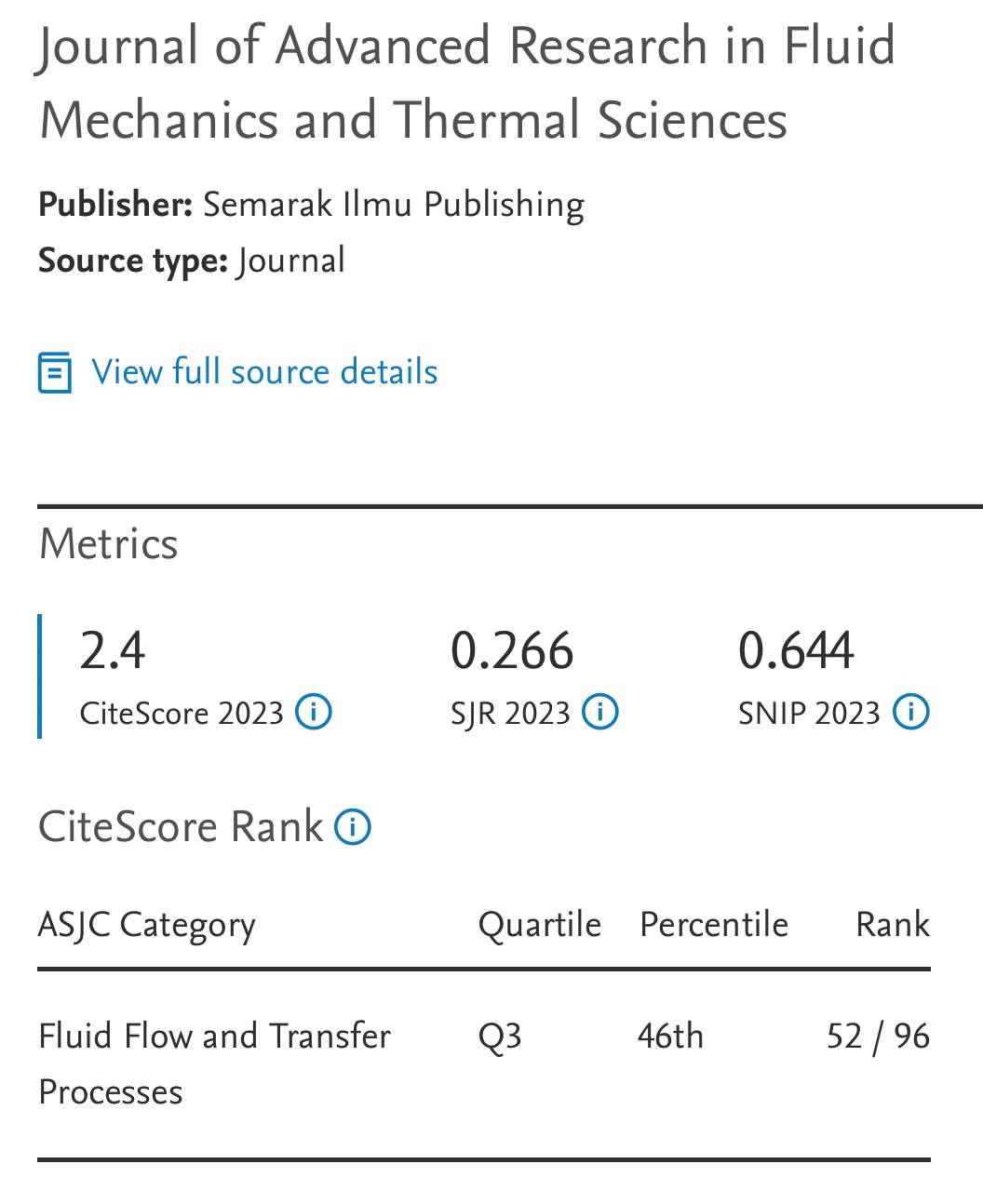
Hydrogen Production by Formic Acid Decomposition with Nanoscale Zero-Valent Iron (nZVI): Effects of nZVI Dosage, Temperature and Time
DOI:
https://doi.org/10.37934/arfmts.125.1.158166Keywords:
Hydrogen, nanoscale zero-valent iron, formic acid, dosage, temperature, timeAbstract
Amidst growing interest in renewable hydrogen gas production, this paper examines three important parameters affecting hydrogen production via formic acid decomposition reaction with nanoscale zero-valent iron (nZVI). The study investigates variations in nZVI dosage (200 – 1000 g/L), reaction temperature (25 – 75°C), and reaction time (5 -30 minutes) to identify optimum conditions for maximum hydrogen yield. Results indicate that the maximum hydrogen yield occurred at nZVI dosage, reaction temperature, and time of 800 g/L, 25°C and 30 minutes, respectively, yielding approximately 215 mL of hydrogen at optimal parameter values. The synthesized nZVI was also analysed before and after the reaction, focusing on the specific surface area and pore size of the nZVI. The results from BET characterization regarding specific surface area and pore size are consistent with experimental results, suggesting smaller pores correspond to higher surface area, enhancing reactivity with formic acid to produce hydrogen gas. Conversely, larger pore sizes after the reaction signify reduced surface area and lower reactivity of nZVI.
Downloads