Liquid-Liquid Equilibria for Ternary Systems Citronellal + Ethanol + Water and Citronellol + Ethanol + Water at 303.15 and 323.15 K
DOI:
https://doi.org/10.37934/arfmts.109.2.231239Keywords:
Citronellal, Citronellol, Liquid-Liquid Equilibrium, NRTL, UNIQUACAbstract
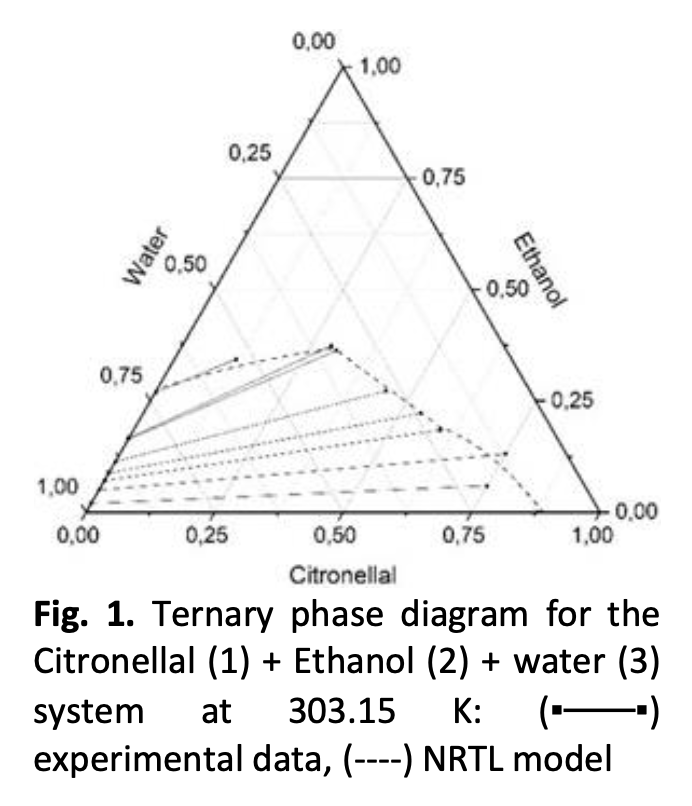
The liquid-liquid equilibria (LLE) for the ternary systems citronellal + ethanol + water and citronellol + ethanol + water are reported. The LLE of the systems have been measured at 303.15 and 323.15 K under atmospheric pressure as a reference for citronella oil production using solvent extraction method. The experimental data obtained were correlated using NRTL and UNIQUAC equations. The non-randomness parameter (αij) of the NRTL model is 0.2-0.47 and fits the experimental data satisfactorily. Both the NRTL and UNIQUAC models gave relatively good results for the systems investigated with the average Root Mean Square Deviation (RMSD) are 0.92% and 0.85% respectively. Based on the ternary diagram obtained in this work, it was found that the system followed the type I classification by Treybal [19]. The binary interaction parameters generated in this study can also be used for LLE calculation in the design of extraction columns.
Downloads