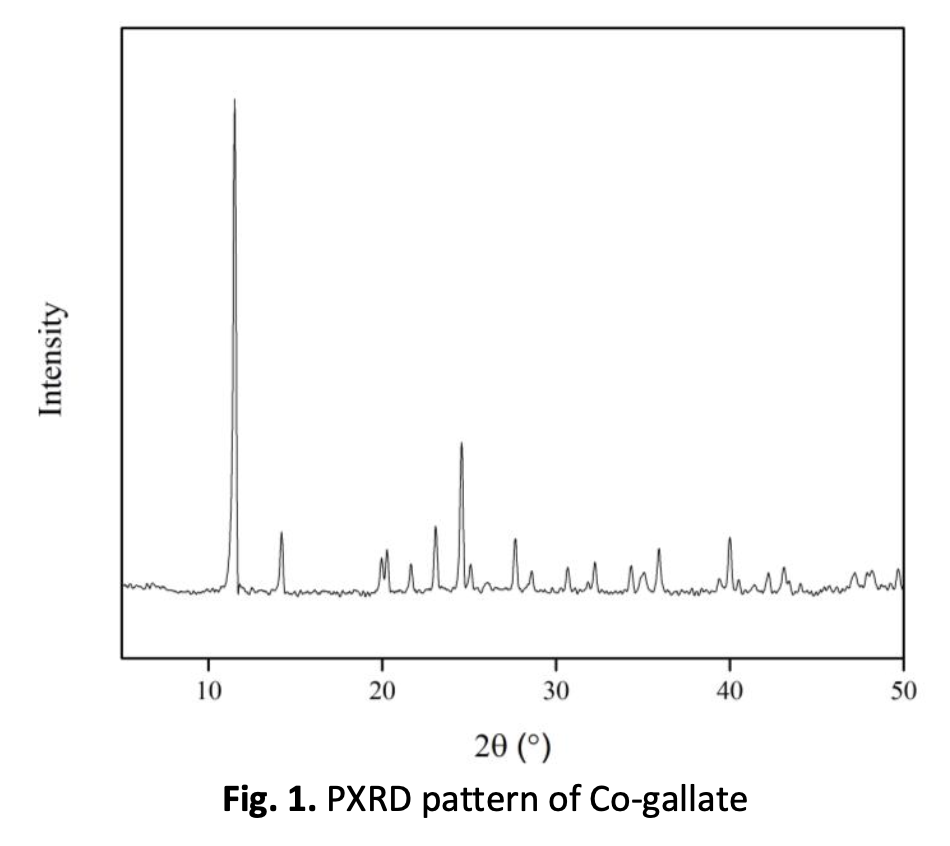
Adsorption of Carbon Dioxide and Methane on Cobalt Gallate-Based Metal-Organic Framework (Co-Gallate): Equilibrium Isotherm, Thermodynamic and Kinetic Studies
DOI:
https://doi.org/10.37934/arfmts.108.2.151163Keywords:
Adsorption, isotherm, thermodynamic, kinetic, MOFAbstract
Biogas is mainly consisted of methane and carbon dioxide in the presence of other contaminants. The biogas purification by adsorption using metal-organic frameworks is getting attention due to the low-cost operation and high-efficiency process. Co-gallate was predicted to give a promising performance in CO2 and CH4 adsorption. However, the behaviours of CO2 and CH4 adsorption on Co-gallate are not well-explained. Therefore, this work is to synthesize Co-gallate and its performance was discussed in terms of adsorbed amount of CO2 and CH4. The experimental CO2 and CH4 pure adsorption isotherms were then fitted with equilibrium isotherm and kinetic models to describe the adsorption behaviours. Co-gallate offered a greater CO2 adsorption capacity than CH4 due to a stronger adsorbent-adsorbate interaction. The experimental pure adsorption isotherms were best fitted with Toth model compared to Langmuir, Freundlich and Sips models according to the values. Toth model described the CO2 adsorption was multilayer and heterogeneous. Thermodynamic property suggested the CO2 and CH4 adsorption were classified as exothermic process and physisorption. For kinetic models, pseudo-first order model brought the highest goodness-of-fit in terms of rate of adsorption compared to pseudo-second order and Elovich models. Pseudo-first order model reflects the adsorption rate is proportional to the number of vacant sites. It confirmed CO2 adsorption was more favourable than CH4, at lower temperature condition. In this work, the equilibrium isotherm and kinetic models were employed to select the best-fitted model in explaining the adsorption behaviours. Therefore, these behaviours of CO2 and CH4 adsorption on Co-gallate are useful in designing the future practical operation of CO2/CH4 gas adsorption.
Downloads