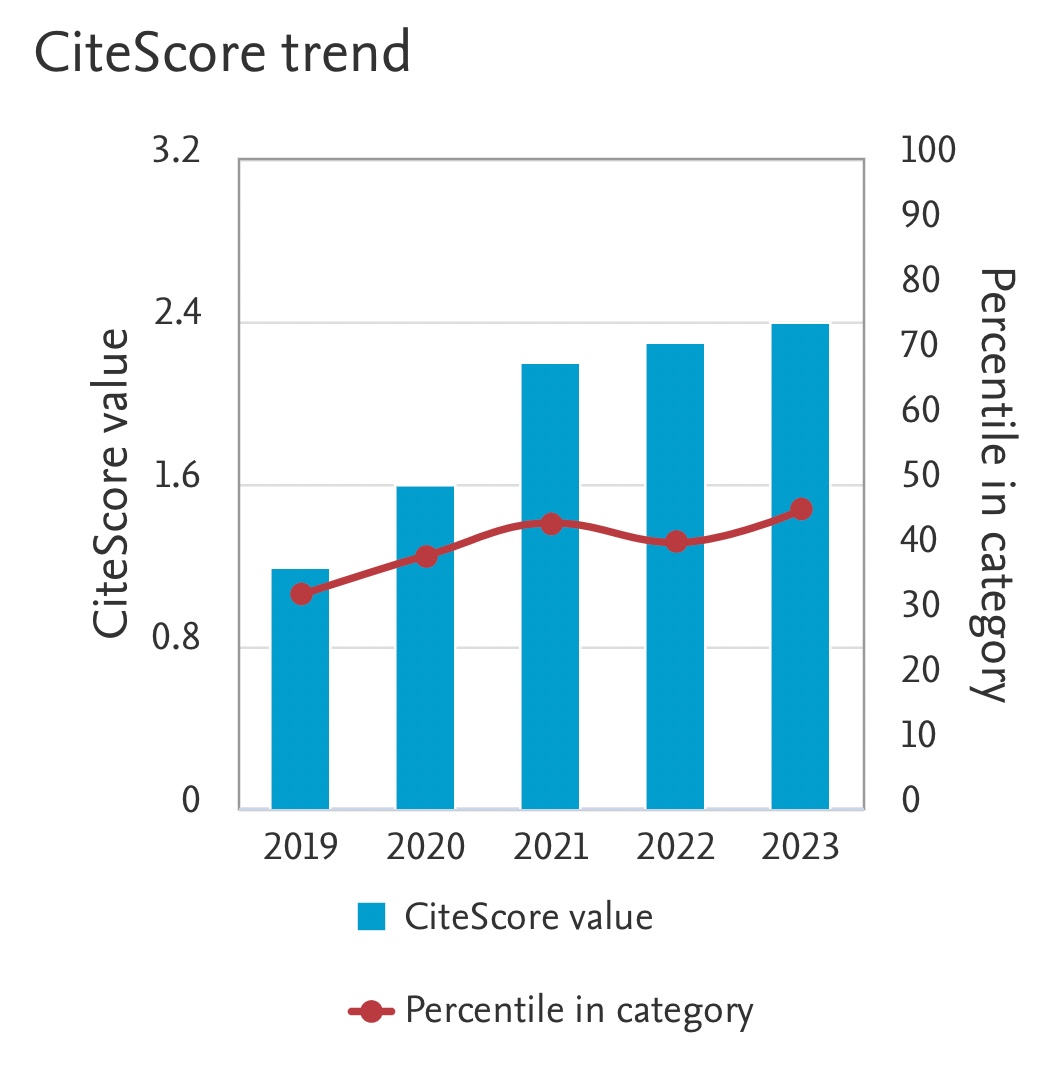
Effect of Different CO2Flow Rate and Calcium Chloride Concentration on CO2Sequestration using Immobilized Carbonic Anhydrase
Keywords:
Sequestration, immobilized, Carbonic anhydrase, CO2, CaCO3, Calcium chlorideAbstract
The present investigation deals with the development of sequestration process of CO2 in carbonic solution accelerated by immobilized Carbonic anhydrase (CA). Sequestration process of the CO2 using biocatalyst has become one of the green and promising approaches as compared to the chemical-based method that cause environmental pollution issue, difficult of the separation and recovery of the solution and also cost ineffective. The present study is focused on the effect of the CO2flow rate and Carbonic solution concentration on the sequestration of CO2 using immobilized CA. The CO2 flow rate was varied from 200 to 800 L/min while, Carbonic solution was prepared using Calcium chloride at 12, 36 and 48 g/L. The results indicate that increase in CO2 flow rate results in a proportional increment of the CaCO3 precipitate. At 200, 500 and 800 L/min of CO2 feeding, amount of CaCO3precipitate was 0.23, 0.26 and 0.30g respectively. On the other hand, the optimum concentration of the carbonic solution was obtained at 36 g/L. The results reveal that at higher CO2flow rate enter the system, the fastest of the equilibrium process can be achieved whereas for carbonic solution concentration, higher concentration of the solution did not significantly affect time for the process to achieve the equilibrium. Time for theprocess to reach equilibrium at CO2 flow rate of 200, 500 and 800 was 14 minutes, 6 minute and 4 minutes respectively. While at different Carbonic solution concentration, the time to reach equilibrium was constantly achieved at 4 minutes for each concentration. The precipitate CaCO3 was validated with XRD analysis and the results indicate a sharp peak that represent the crystal structure of CaCO3 at 2θ = 23.07, 29.42, 36.0, 39.43, 43.3, 47.53, and 48.53. The morphology of the precipitate observed using scanning electron microspore showed a rectangular shape of the CaCO3. From the study, the optimum carbonic solution concentration was 36 g/L and the CO2 loading can be speed up to 800 L/min to achieve a faster sequestration process.
Downloads