A Kinetic Study on Tetraselmis chuii Combustion: The Catalytic Impact of Nanoparticle Titanium Dioxide (TiO2) Additive
Keywords:
Microalgae, Tetraselmis chuii, Titanium dioxide, Kinetic parameters, Thermodynamic parametersAbstract
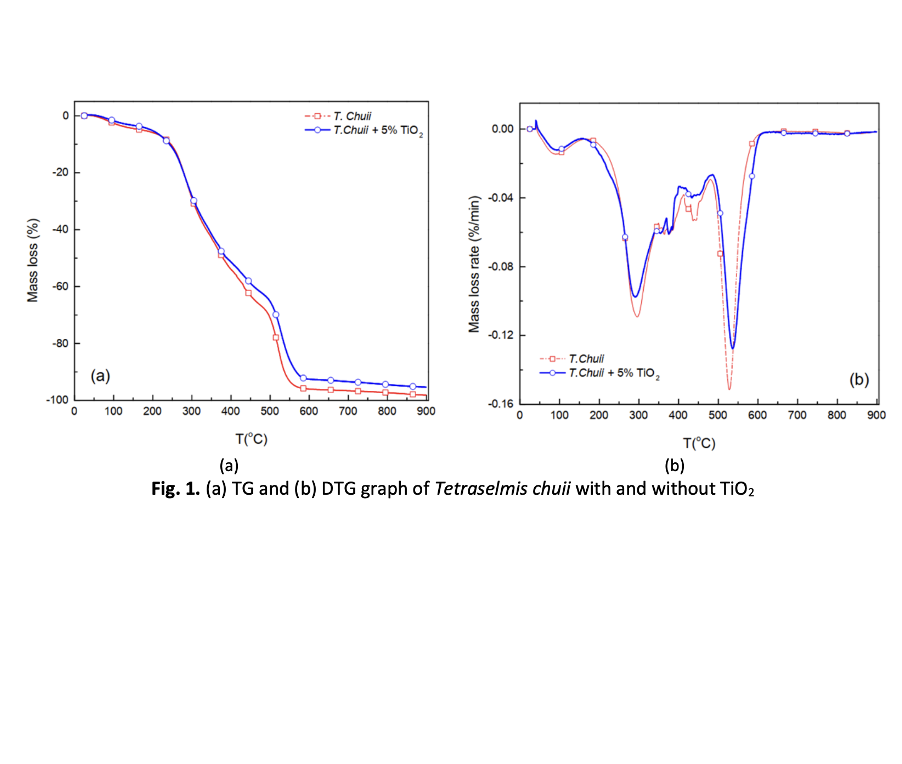
Due to the depletion of fossil fuel reserves and the increasing energy demand, biomass is a promising alternative as an alternative energy source. This study aims to investigate the catalytic effect of Titanium dioxide (TiO2) nanoparticles on the thermal behavior and kinetic parameters of Tetraselmis chuii (T.chuii) microalgae during the combustion process in the thermal analyzer. TiO2 amount of 0.05 mg was mixed with 10 mg microalgae biomass. The sample was heated up from room temperature until 900 oC with a heating rate of 15 oC/min and an atmosphere flow rate of 50 mL/min. The results indicated that the sample was decomposed into five stages throughout the escalation temperature. The addition of TiO2 promoted each stage of decomposition toward the lower temperatures and shorter the combustion time. The activation energy value was analyzed using the Arrhenius method. The TiO2 nanoparticle impacted in reducing the value of activation energy in stage II from 101.05 kJ/mol to 72.07 kJ/mol, and stage IV from 502.88 kJ/mol to 335.32 kJ/mol. The thermodynamic parameters included entropy, enthalpy and free Gibbs energy of mixed samples in stage II were -0.1396; 67.41 and 146.14 kJ/mol, respectively, and in stage IV were 0.1541; 328.31 and 203.68 kJ/mol, in that order. These results are practically proven that the addition of TiO2 nanoparticles have been shortening the thermal process and reducing the value of activation energy.
Downloads