Adsorption of Pb (II) Ions in Water with Natural Kenaf Beads
DOI:
https://doi.org/10.37934/araset.32.1.179187Keywords:
Kenaf, polluted, heavy metals, adsorption, Sustainable Development GoalsAbstract
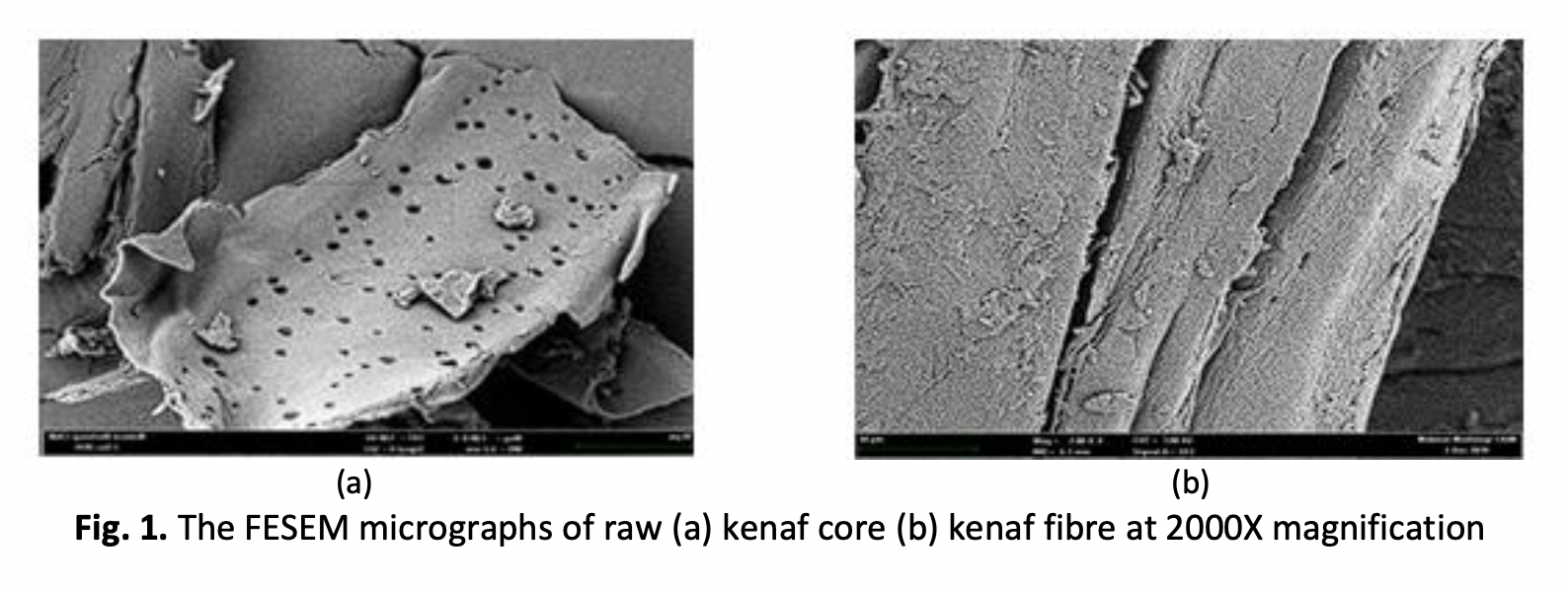
The purpose of this research is to study plant-based kenaf adsorbents on the adsorption of polluted Pb (II) in aqueous solutions. CHT and KNF core powder were added to the sodium alginate solution and stirred homogeneously. The blend solution was dripped into calcium chloride to form smooth magnetic KNF-CHT-ALG beads. The functional group presence in the beads and percentage adsorption of the heavy metals were checked using Fourier Transform Infrared Spectroscopy (FTIR) and Inductively Coupling Method analyses, respectively. It was found that the surface morphology of kenaf core was rougher than that of kenaf fibre, with the presence of a larger amount of micropores as observed in the FESEM analysis. In addition, the FTIR pattern of the KNF-CHT-ALG beads had shown the existence of functional groups such as hydroxyl and carboxyl, which could attract more positively charged heavy metals. The ICP analysis confirmed the successful 95% adsorption of the heavy metals. Kenaf is an abundant crop available in Malaysia that may reduce the production cost of the adsorbents. The significant outcomes of this study would be its contribution to minimising the dependency on the chemical adsorbent and to accelerating the removal process of heavy metals in real water bodies. Thus, this study aims to create a more sustainable future, especially by reaching SDG 6: Clean Water and Sanitation.
Downloads