Isotherm, Characterisation and Regeneration Studies for the Adsorption of Pb(II) Ions in Water
DOI:
https://doi.org/10.37934/araset.29.3.175184Keywords:
Regeneration, KENAF adsorbent, heavy metals, isotherm, characterisationAbstract
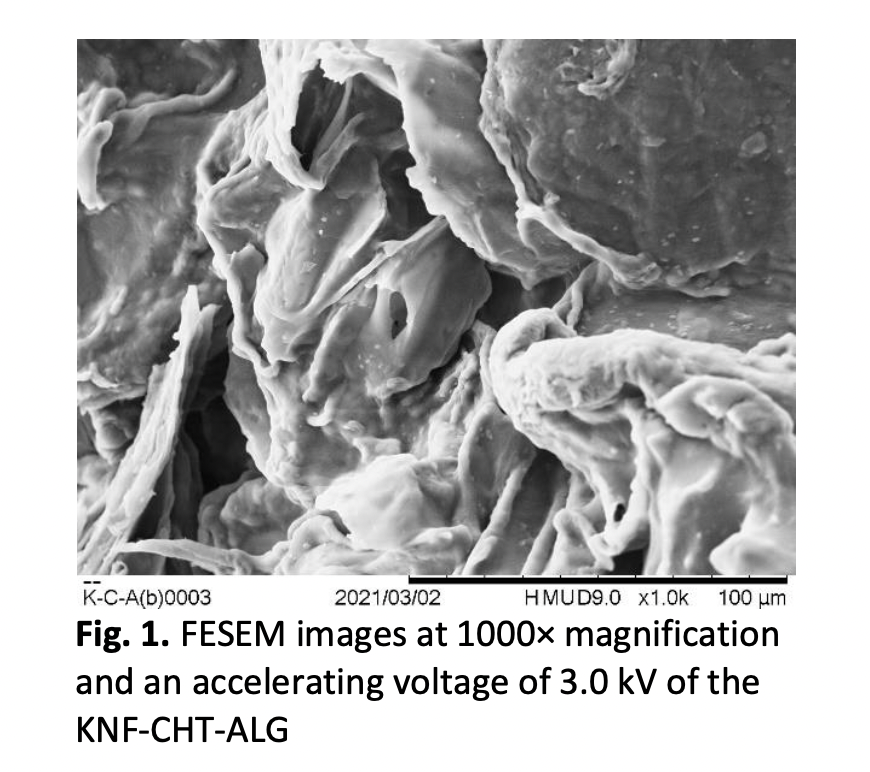
The adsorption isotherms and regeneration study of the removal of Pb(II) ions from water using kenaf-chitosan-alginate (KNF-CHT-ALG) beads were evaluated. The effects of initial Pb(II) ions concentration on the adsorption capacity of KNF-CHT-ALG beads were carried out in a batch study mode and analysed using the Inductively Coupled Plasma (ICP) technique. In the present research, the linear models of Langmuir and Freundlich were used to predict the adsorption isotherms. The adsorption process was excellently well fitted with the Langmuir isotherm model. The maximum adsorption capacity recorded 33.557 mg/g. For the regeneration study, after five times of the recycling process, the KNF-CHT-ALG beads still showed good adsorption towards Pb(II) ions with maximum removal of 95% and regeneration of 98%. Clearly, from the research conducted, the KNF-CHT-ALG beads are found to be a suitable adsorbent for Pb(II) ions removal.
Downloads