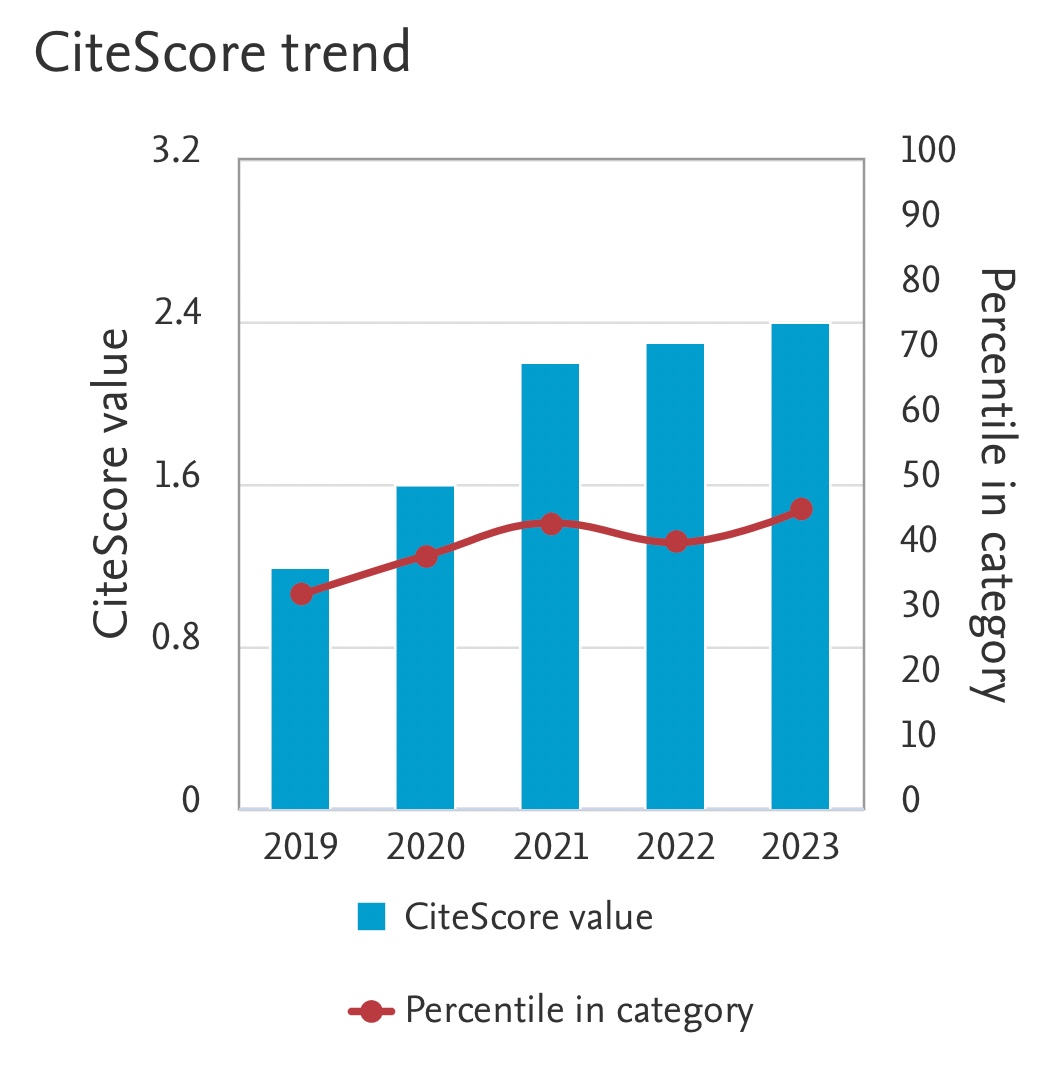
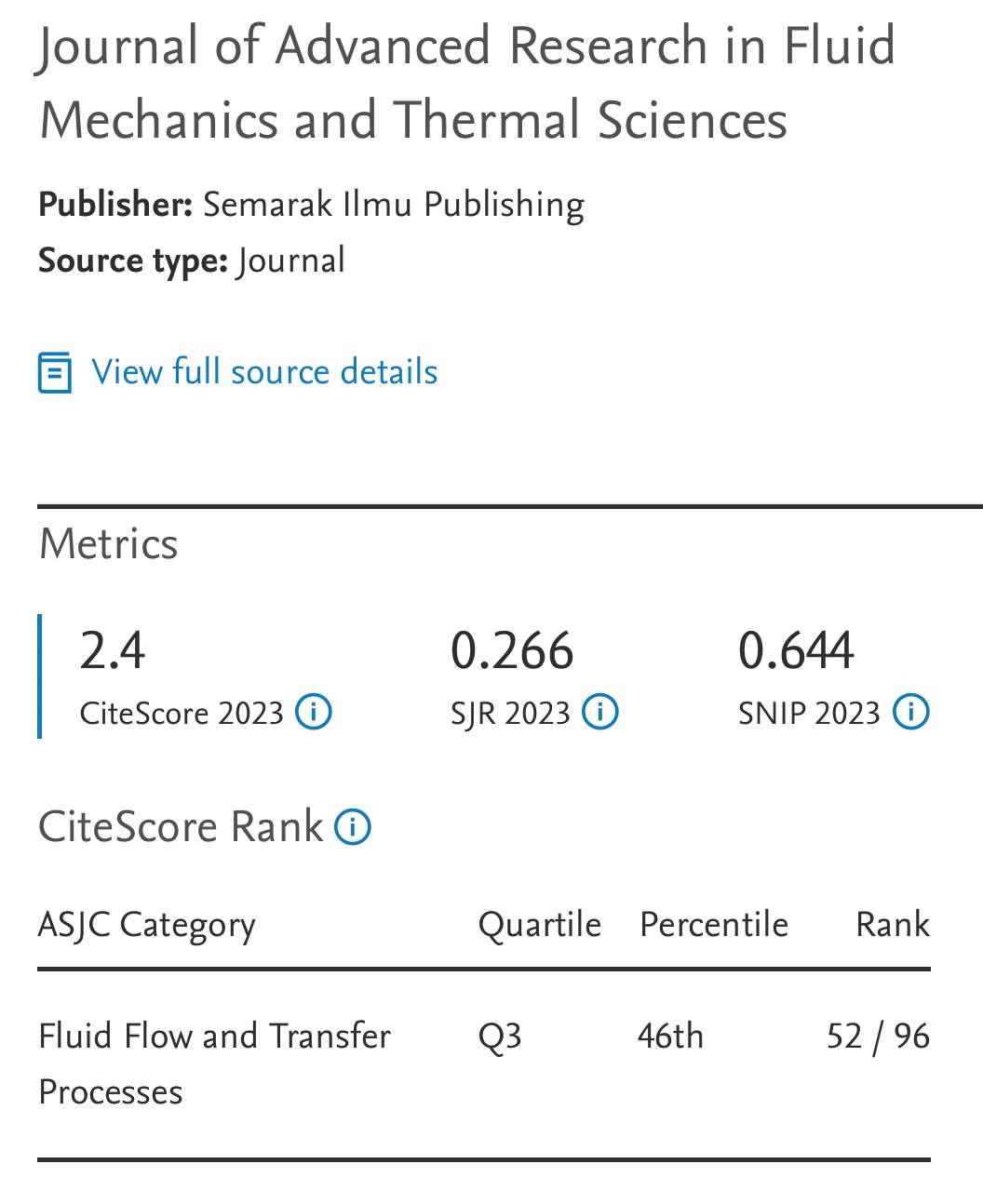
Investigation of Carbon Dioxide Solubility in Aqueous N-methyldiethanolamine (MDEA)-1- Butyl-3-Methylimidazolium Acetate ([bmim][Ac]) Hybrid Solvent
Keywords:
Carbon dioxide capture , hybrid solvent , Ionic liquids , solubilityAbstract
In this research work, aqueous hybrid solvents were prepared at different concentrations of 1-butyl-3 methylimidazolium acetate, [bmim][Ac] as ionic liquids (ILs) and N-methyldiethanolamine (MDEA) for the capturing of carbon dioxide (CO2). The concentration of MDEA was kept constant at 30 wt% in every hybrid solvent investigated. The solubility of CO2 in the hybrid solvent was investigated by varying the concentration of [bmim][Ac] in the aqueous hybrid solvent as 10 wt% and 20 wt%. For comparison, the solubility experiment was also conducted for pure aqueous MDEA solvent. Addition of [bmim][Ac] in the hybrid solvent reasonably enhanced the solubility of CO2 as compared to its solubility in pure aqueous MDEA solvent. The loading capacity of hybrid solvent was also improved significantly with hybrid solvents. Further increasing the concentration of [bmim][Ac] from 10 wt% to 20 wt% has demonstrated a decrease in the solubility of CO2 into the hybrid solvent. The effect of pressure on loading capacity and rate of absorption was also investigated by increasing the pressure from 10 bar to 20 bar. Both loading capacity and rate of absorption increased with increasing pressure. Promising results were achieved using hybrid solvents for capturing of CO2
Downloads