Preliminary Study of Novel Coal Spills-based Physical Activated Carbon for Copper ions Adsorption in Aqueous Solution: Kinetics and Isotherms
Keywords:
Coal spills, activated carbon, adsorption, copper, kinetics, isothermsAbstract
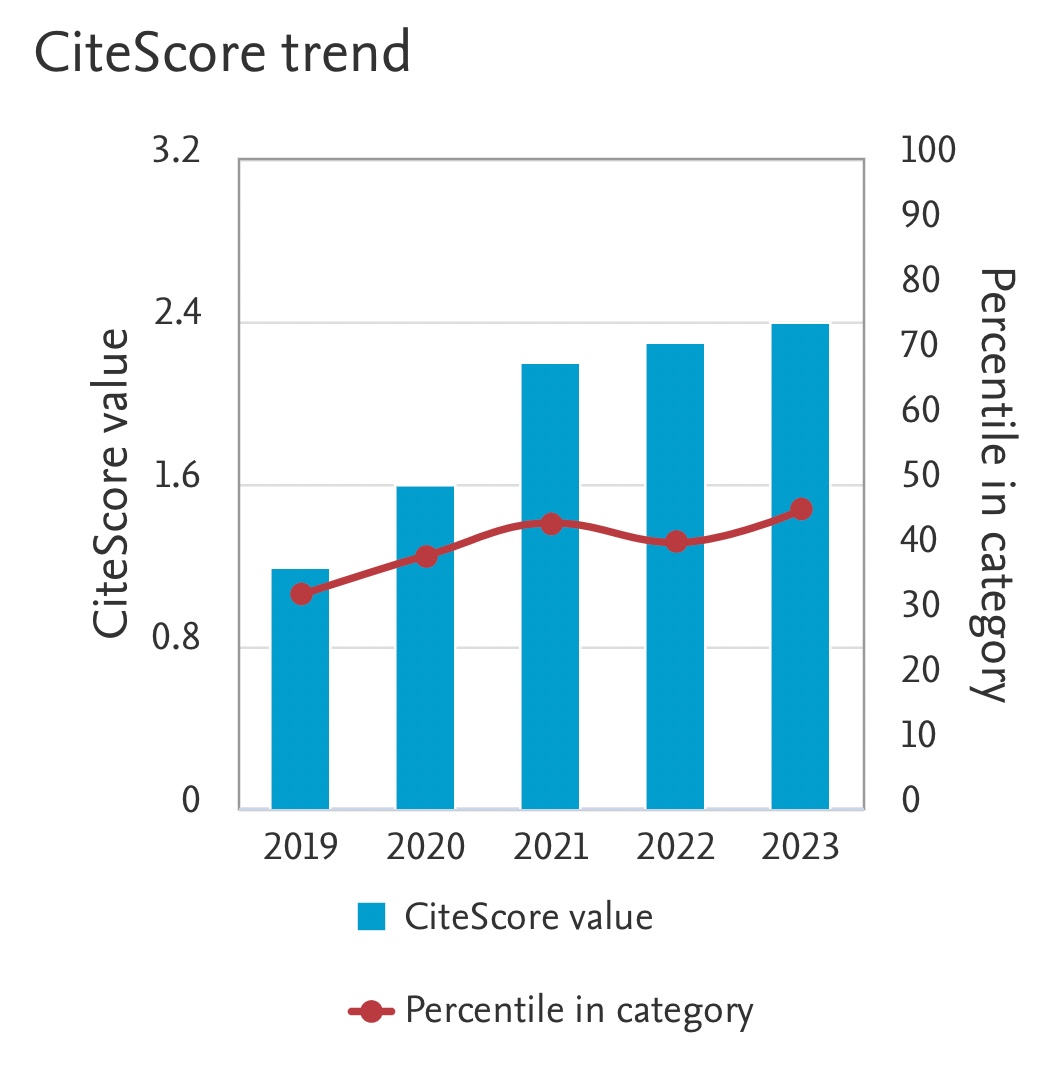
This study proposed a novel activated carbon which was prepared from coal spills by physical activation. It was activated in tube furnace at 500 °C with nitrogen injection. Based on Fourier Transform Infrared Spectroscopy and Scanning Electron Microscopy analyses, the coal spills-based activated carbon (CSFAC) was expected to have more pores and cavities compared to the untreated coal spills (UCS). This preliminary study focused on adsorption kinetics and isotherms by investigating separately the effects of two independent variables i.e. contact time and initial Cu(II) ions concentration. The temperature of ion solution was set at 30 °C (1 atm) and initial pH 5. The Cu (II) ions adsorbed onto the CSFAC and USC had best fitting to the pseudo-second-order kinetics model with R 2 being 1.000 and 0.978, respectively. The Cu(II) ions equilibrium adsorption capacity and adsorption rate of the CSFAC were 90.909 mg/g and 0.093 g/mg.min respectively and they tracked well Freundlich adsorption isotherms with R 2 being 0.811 and 0.917, respectively. The Freundlich-based pore volume and adsorption intensity were 3.861 L/mg and 1.132 respectively. The Brunauer-Emmett-Tellersurface area and total pore volume of the CSFAC were approximately 50.848 m²/g and 0.018 cc/g respectively. Based on this research, CSFAC was found to be a good potential candidate to be used in water treatment in the near future.
Downloads