Polarization Curve of Chloride-Induced Corrosion in Steel Using a Three-Dimensional Model
DOI:
https://doi.org/10.37934/aram.106.1.2736Keywords:
Corrosion, steel, modelAbstract
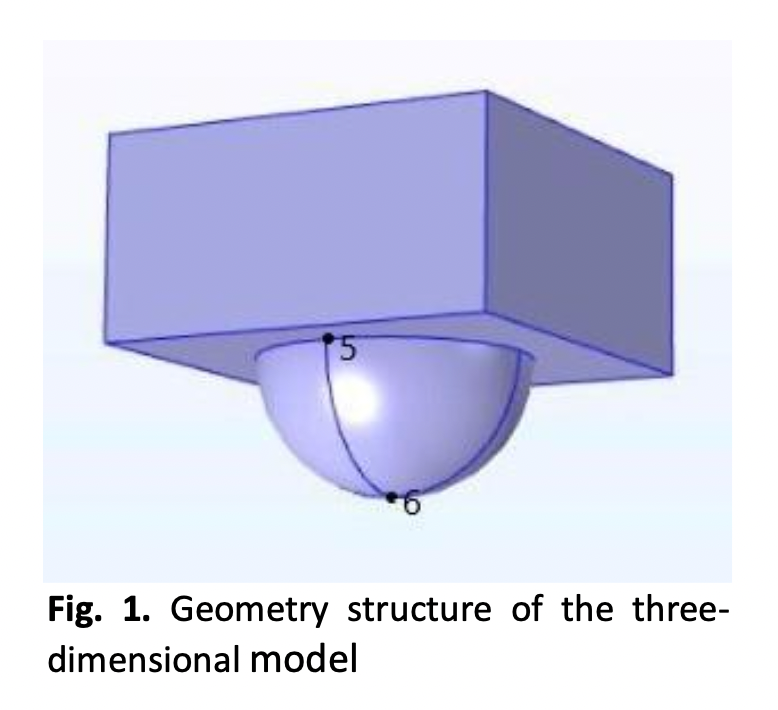
Corrosion process occurs naturally in which metals, such as steel, undergo degradation due to chemical or electrochemical reactions with their surroundings. This however can lead to serious malfunctions and costly damages to crucial steel uses. In marine activities, most metals and alloys used inevitably degrade since the sea is rich in corrosive electrolytes, in particular, chloride. This may lead to the occurrence of pitting corrosion, which is highly localised and can cause major catastrophes. Hence, it is important to understand the science of pitting corrosion which in turn can benefit corrosion control processes. This can be done through simulation of pitting activities. In this study, a three-dimensional model of a minute pit in steel, immersed in sodium chloride solution in water, is simulated using COMSOL Multiphysics version 5.6 with Chemical Reaction Engineering Module. The simulation is run to observe the shape of the polarization curve at the actively corroding pit. The initial pH of the immersing solution is at a slightly alkaline condition, namely at pH 8. The results show that the corrosion potential, Ecorr, of a point at the bottom of the corroding pit is -0.74 V. The model is also able to show a high concentration of ferrous ions, Fe2+, inside the pit geometry, reaching to a value of 2.5×103 mol m-3. These results indicates that metal dissolution occurs. The shape of the polarization curve obtained in this study exhibits a significant linkage to the propagation of pit, which in turn indicates the type of corrosion a pit can evolve into, in particular, pitting corrosion.
Downloads