Synthesis and Characterization of Activated Carbon Derived from Rubberwood Sawdust via Carbonization and Chemical Activation as Electrode Material for Supercapacitor
DOI:
https://doi.org/10.37934/arfmts.94.2.6176Keywords:
Activated carbon, chemical activation, electrode material, rubberwood sawdust, supercapacitorAbstract
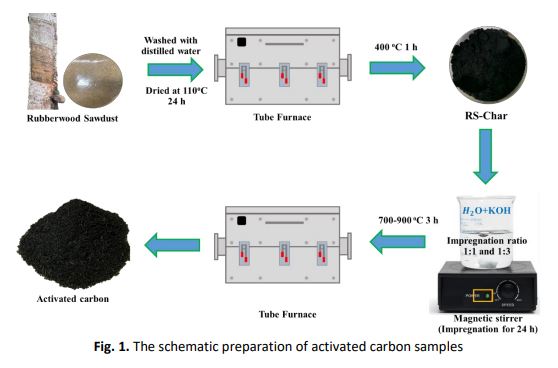
This research aims to study the physical and electrochemical properties of the activated carbon derived from rubberwood sawdust as electrode material for supercapacitors. Rubberwood sawdust, waste from the wood processing industry, was used as the precursor for synthesizing activated carbon using potassium hydroxide (KOH) as an activation agent. The carbonization was carried out at 400 °C under the nitrogen atmosphere. In this experiment, the rubberwood sawdust chars were activated by impregnating KOH solution using the impregnation ratios 1:1 and 1:3 (Char: KOH). After that, the samples were activated at 700, 800, and 900 °C, respectively. The adsorption isotherm, surface area, total pore volume, pore size distribution, and structure porosity were investigated. The XRD was used to examine the structural characteristics. The morphology of the sample was characterized by FESEM. The surface functional group of the samples was determined by FTIR and Raman spectroscopy. The three-electrode testing consists of the activated carbon samples coated on aluminum foil (Working electrode), Ag/AgCl (Reference electrode), and platinum wire (counter electrode) in 1 molar of KOH. According to the research, the activated carbon at an impregnation ratio of 1:3 with an activation temperature of 900 °C presented the highest surface area, total pore volume, and average pore diameter as 1,574.39 m2/g, 0.6906 cm3/g, and 1.89 nm, respectively. The cyclic voltammetry (CV) result showed a high specific capacitance value of 54.08 F/g at a scan rate of 5 mV/s in the case of the impregnation ratio of 1:3 with an activation temperature of 900 °C. Thus, the activated carbon derived from rubberwood sawdust waste as an electrode material can be a promising candidate for supercapacitors due to its outstanding electrochemical properties.
Downloads