Quartz Hydrolysis Analysis of Pure Quartz for Enhanced Oil Production; Influence of Time, pH, and Salinity on Hydrolysis
DOI:
https://doi.org/10.37934/arfmts.111.2.99106Keywords:
Silica dissolution, sand production control, water breakthrough, semi-consolidated formationAbstract
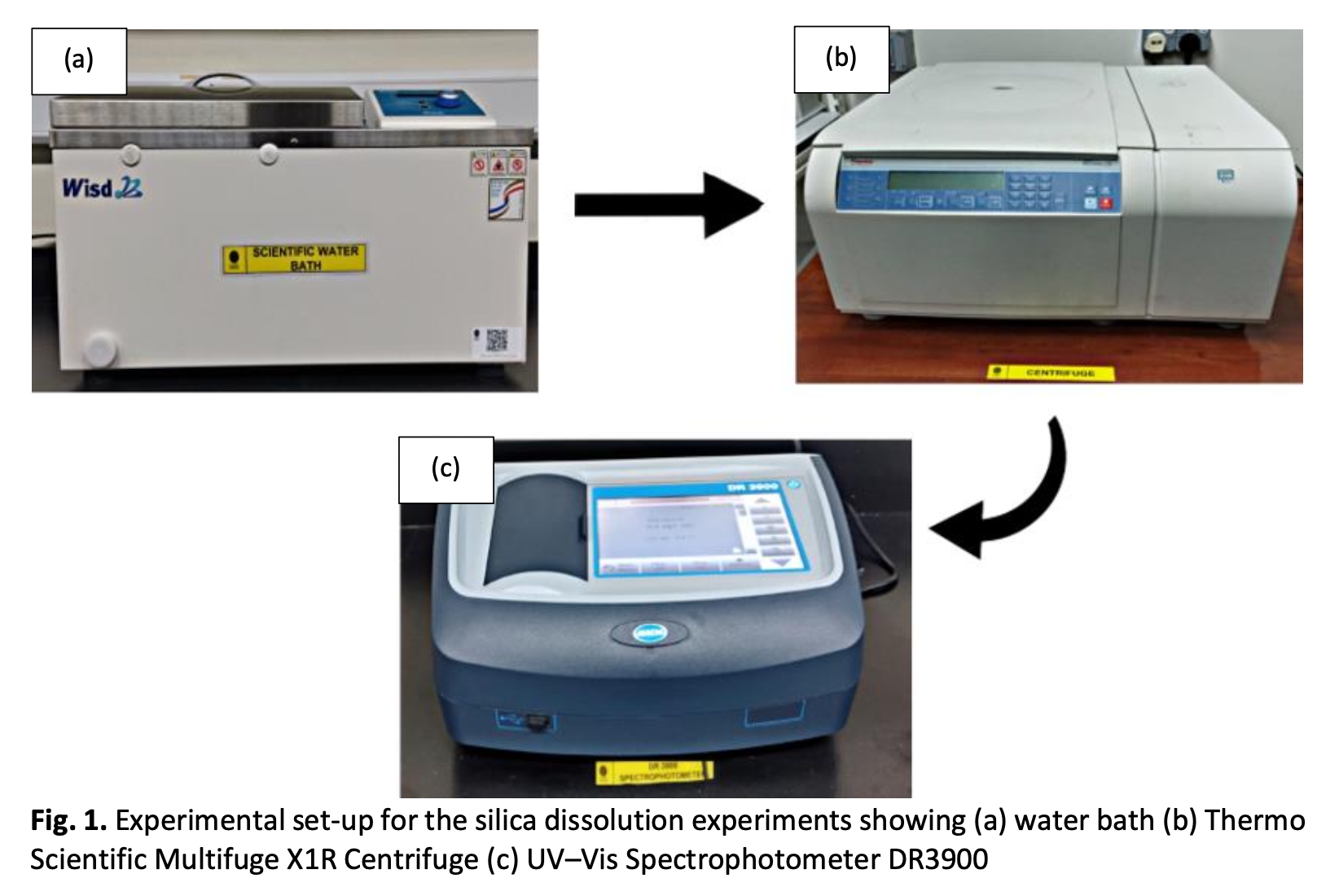
The issue of sand production in reservoirs has been recognized as a critical problem in oil and gas fields for several years due to its numerous negative impacts on oil and gas production. Sand production from wells can damage both surface and subsurface equipment, reduce well productivity, and negatively impact the oil production economy. Oil production challenges arise when sand production increases as the water cut increases. Several factors have been associated with this phenomenon, with the most critical factor being silica dissolution - a chemical reaction between quartz and water that decreases formation strength. This study explores the dissolution of silica on pure quartz and the effects of added electrolyte (NaCl, CaCl2, MgCl2, and KCl) concentration, temperature, and pH on the dissolution process. The concentration of silica dissolved in solution was measured using UV-Vis spectrophotometry to determine the dissolution of silica on quartz. The results outcome indicate that the rate of silica dissolution is significantly influenced by variations in temperature and alterations in pH levels. The salinity conditions exhibit minimal alterations in comparison to distilled water. The highest concentration of silica dissolved in a solution has been seen at pH levels of 12 and 3, with concentrations of 118 mg/l and 68 mg/l, respectively. In terms of temperature, it was observed that there was an increase in the dissolution of silica from 30°C to 90°C, ranging from around 170% to 600%. Regarding salinity, it can be shown that NaCl and KCl exhibit the most significant impact on silica dissolution when compared to other brine solutions, with concentrations of 45 and 49 mg/l, respectively. The study claims that the formation strength may be influenced by water quality through the processes aimed at stimulating or lowering silica dissolution. Therefore, the optimal water quality design for water injection is paramount in mitigating sand production challenges.
Downloads
Downloads
Published
How to Cite
Issue
Section
10.1016/j.biortech.2024.131961
































