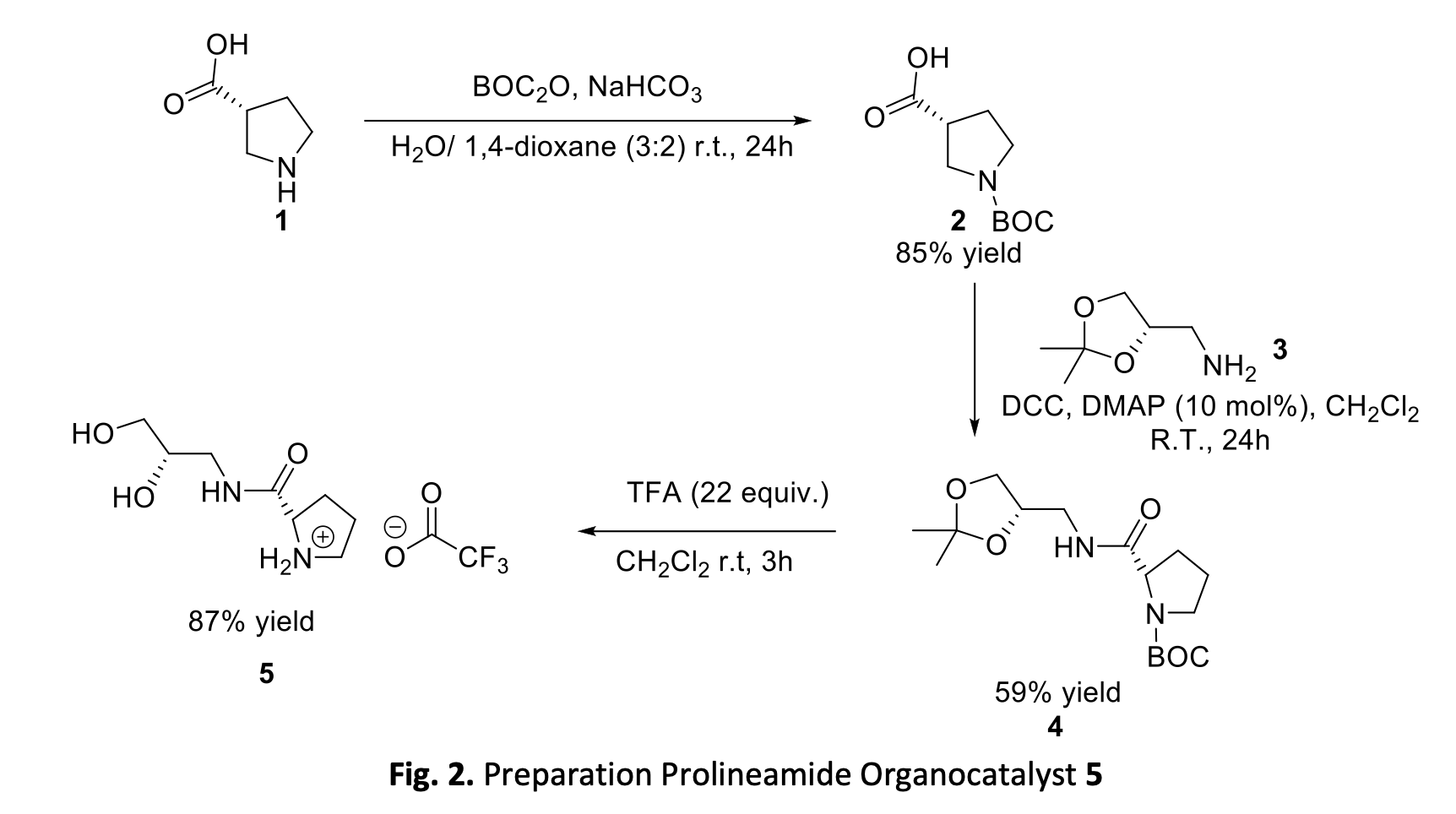
Synthesis of Trans-4-Hydroxyprolineamide and 3-Ketoproline Ethyl Ester for Green Asymmetric Organocatalysts
DOI:
https://doi.org/10.37934/araset.38.1.97108Keywords:
Organocatalysts, Proline, Chiral Prolineamide, Condensation Reaction, Pyrrolidine RingAbstract
Organocatalysts have become one of the three pillars in asymmetric reactions, along with metal catalysis and enzyme catalysis. Organocatalysis is widely acknowledged in both academia and industry as a practical and advantageous synthetic method owing to its operational ease, readily available catalyst, environmentally friendly, and minimal toxicity. Much attention has been focused on the organocatalyst for its superior properties as an efficient and clean catalyst. In this work, a series of green organocatalysts of trans-4-hydroxyprolineamide were efficiently obtained in a two-step reaction utilizing EDC.HCl and HOBT as coupling reagents via a condensation reaction. The yield furnished in 93% to 97% yields. These organocatalysts have big potential in asymmetric reactions such as aldol and Michael addition reactions.
Downloads