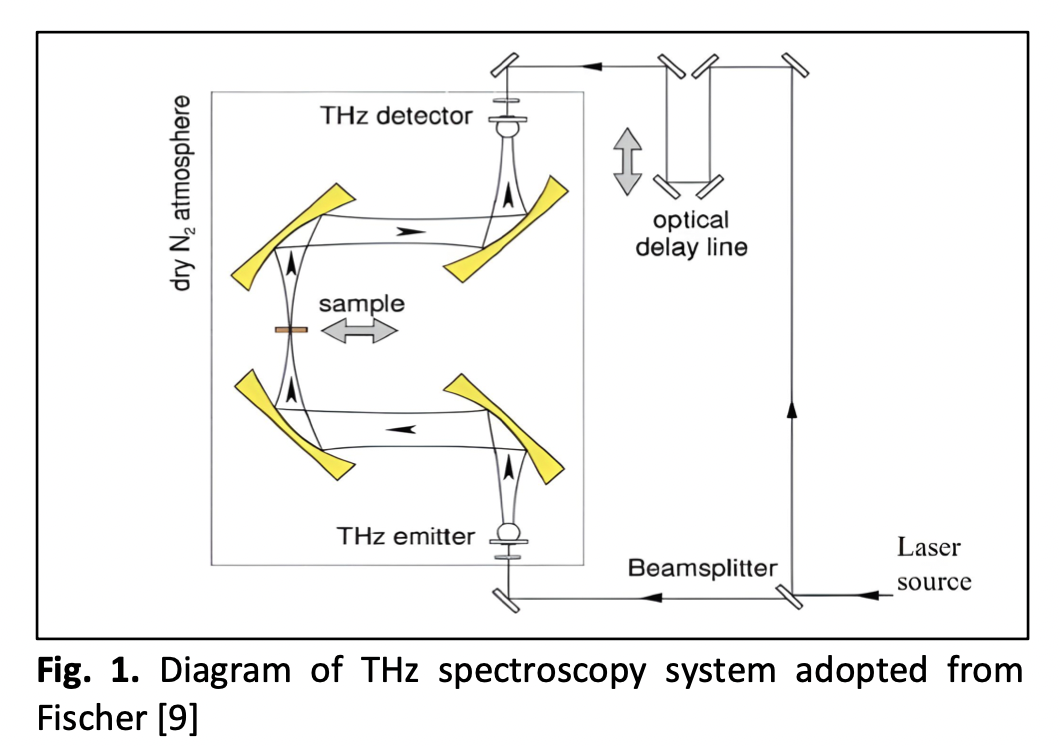
Terahertz Spectroscopy Analysis of Amyloid Fibrils Derived from Human Serum Albumin
DOI:
https://doi.org/10.37934/araset.43.1.160170Keywords:
Amyloid disease, protein fibrils, human serum albumin, Terahertz time domain spectroscopy (THz-TDS)Abstract
Amyloid fibrils, also referred to as protein fibrils, represent irregular assemblies of proteins exhibiting a cross-β configuration. Terahertz radiation, a segment of the electromagnetic spectrum spanning 0.3 to 10 terahertz (THz), is known to interact with these fibrils due to their cylindrical shape. This study's objective was to explore the potential of terahertz spectroscopy as a non-invasive means to detect the progressive growth of protein fibrils. In this investigation, human serum albumin (HSA) was synthetically induced to form protein fibrils. Confirmation of fibril formation was done by fluorescence spectroscopy, Fourier transform infrared (FTIR) spectroscopy, UV-Vis spectroscopy, and Terahertz time-domain spectroscopy (THz-TDS). To initiate the process, HSA was dissolved in distilled water at a concentration of 160 µM, followed by the addition of 60% ethanol to both samples for dilution. The HSA to Cu (II) ratio was maintained at 1:1 (160 µM) for stock solutions containing Cu (II). Subsequently, each set underwent a 6-hour heating phase at 65 °C to cultivate fibrils, which were then stored at room temperature for 30, 60, and 90 days before evaluation. Incubation of human serum albumin yielded amyloid fibrils as evidenced by ThT fluorescence, FT-IR spectroscopy revealed an emergence of fibrils within the amide I bands, extending from 1630 cm⁻¹ to 1650 cm⁻¹ and UV-Vis spectroscopy disclosed the augmentation of protein fibril absorbance over the incubation period. THz spectroscopy absorbance progressively heightened with prolonged heating of the protein fibrils due to the hydration shell surrounding them. Through these spectroscopic methods, it was elucidated that the fibrillation process led to the gradual development of β-sheet and unordered helix structures as well as the potential of THz spectroscopy as non-invasive tool for detection and monitoring of amyloidal diseases, promising insights into early diagnosis and treatment strategies.
Downloads