Peng-Robinson Cubic Equation of State Based on Key Group Contribution and Calculation of Nitrogen Gas Solubility in MMA Dimer
DOI:
https://doi.org/10.37934/arfmts.88.1.111Keywords:
Gas solubility, vinyl monomer, group contribution, Peng-Robinson equation of state, scalingAbstract
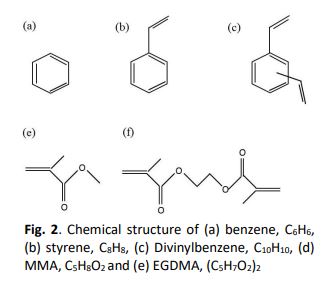
Previously authors have reported facts that nitrogen (N2) and oxygen solubilities in styrene is the same as those in benzene and divinylbenzene at 303 K. Though the three compounds have an atomic composition, (CH)n (n=6, 8 and 10), the gas solubilities are thought to depend on the number of aromatic carbons in the solution. Then, authors named it ‘a key group in solubility’. In this research, the key group was investigated for N2 solubility in methyl methacrylate (MMA) and its dimer, ethylene glycol dimethacrylate (EGDMA). Therefore, the calculations were carried out by Peng-Robinson (PR) equation of state based on group contribution methods. The experimental data employed were those of Lai et al., where N2 solubility are reported in MMA at 303 K. The N2 solubility in EGDMA was assumed to be double of that in MMA at 303 K, because EGDMA has two MMA units in the molecule. Two types of group contribution methods were proposed to calculate N2 solubility in EGDMA. One was for the critical temperature and pressure proposed by Joback and Reid. Using these properties, the attractive and excluded volume parameters in PR equation were evaluated for EGDMA. Other was similar to a method proposed by Orbey and Sandler. The excluded volume parameter in PR equation can be evaluated from those of MMA by multiplying the number of repeating units, and the attractive by multiplying the squared number. Instead of the method, the powers were proposed to be 3/4 and 3/2 for the excluded volume and attractive perimeters, respectively. The powers were come from a scaling theory by de Gennes. The binary parameter for N2 – EGDMA was also set to k12 = 0.224, which was the same as that for N2 –MMA. The calculated N2 solubility in EGDMA was far smaller than the hypothetical N2 solubility in EGDMA at 303 K. Otherwise, to reproduce the hypothetical N2 solubility, a quite different value, k12 = -0.044, was necessary for the method by Joback and Reid. The results suggested that a key group for gas solubility will not be accepted in MMA.
Downloads