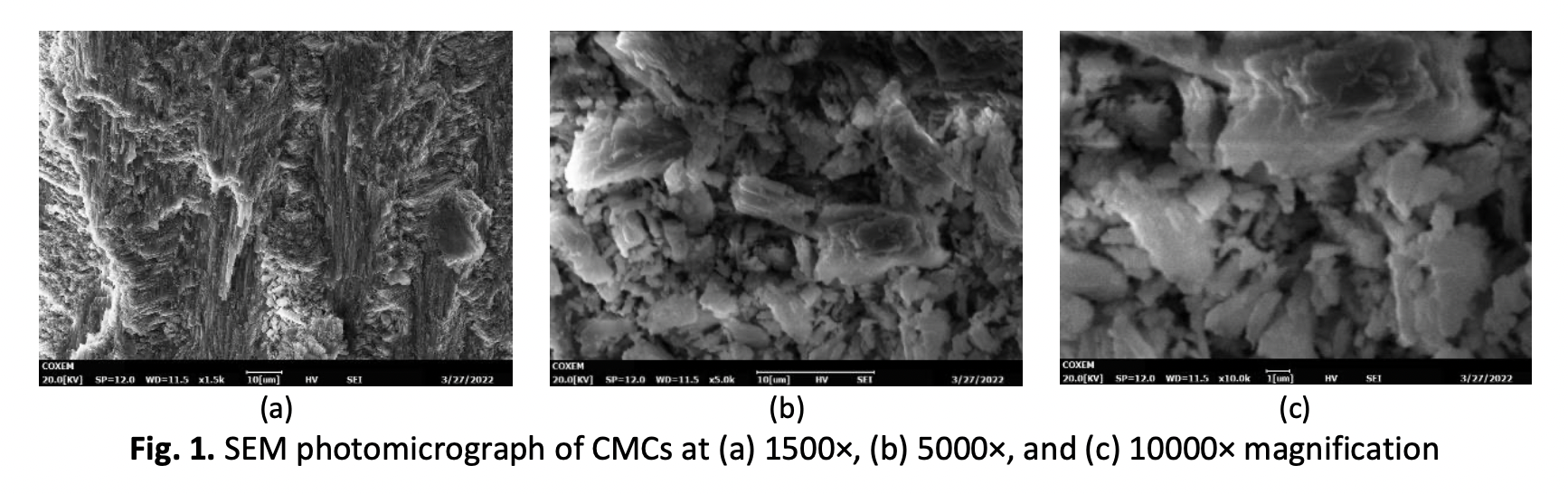
Effect of Various Sizes of Calcined Marsh Clam Shell on Phosphate Removal from Aqueous Solution
DOI:
https://doi.org/10.37934/arfmts.113.1.95107Keywords:
Phosphorus, eutrophication, marsh clam shell, adsorption, kinetic, isothermAbstract
Phosphorus is one of essential elements for sustaining life largely through phosphate, a compound containing the phosphate ion, PO43−. However, excessive phosphorus concentrations will cause eutrophication and this condition is considered as an environmental issue. The phosphorus sources are commonly from human activities; for instance, detergents, fertilisers, and industries. Although various adsorption techniques have been applied to remove phosphorus in water, the effect of different particle sizes of calcined marsh clam shells on phosphate removal has not been fully investigated, along with its adsorption kinetics and isotherms. This study investigates phosphate removal from synthetic solution onto calcined marsh clam shells (CMCs) with 5 different particle sizes: 0.075–0.15, 0.15–0.30, 0.30–0.60, 0.60–1.18, and 1.18–2.36 mm. The batch experiment used 2 g of adsorbent and synthetic potassium dihydrogen phosphate (KH2PO4) solution. The adsorbent size of 0.075 to 0.15 mm showed the highest removal (100%) efficiency due to high adsorption rate. The experimental data were further analysed using kinetics and isotherm models; the data fitted well with pseudo-second-order kinetic model (R2 = 0.9986) and Freundlich isotherm model (R2 = 0.8404). The potential of marsh clam shells as an adsorbent for phosphate removal in water is significant for future applications in wastewater treatment technology.
Downloads