Biodiesel Production from Canola Oil Using TiO2CaO as a Heterogenous Catalyst
DOI:
https://doi.org/10.37934/arfmts.93.2.125137Keywords:
Biodiesel, Canola Oil, TiO2, CaO, Production, Vegetable Oils, TiO2/CaOAbstract
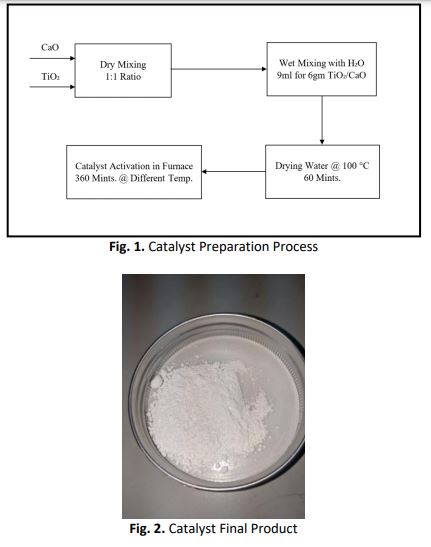
Biodiesel is one of the renewable energy sources that is an alternative to fossil diesel that is non-toxic and produces less CO emissions. Transesterification process is a conventional mechanism to produce biodiesel from vegetable oil with a homogeneous or heterogenous catalyst. However, heterogenous catalysts are considered as more efficient than homogenous catalysts. Recently, TiO2/CaO has been used as a compound heterogenous catalyst to produce biodiesel produce from palm oil, waste cooking oils and algae. In this research, biodiesel was manufactured using canola oil as a feedstock and titanium dioxide / calcium oxide (TiO2/CaO) as a catalyst. The aim of this study is to prepare the catalyst, investigate the transesterification process and measure the chemical and physical biodiesel properties. Catalyst preparation required four stages: dry mixing, wet mixing, water separation and catalyst activation where there were two temperature phases (200 °C and 600 °C). Catalyst mixed with methanol by 1:16 ratio had different mixing time phases (30 minutes, 60 minutes, and 90 minutes). The Transesterification process was by blending the catalyst-methanol mixture with canola oil under 3 phases (4 hours, 5 hours, and 6 hours). The catalyst characterization was by analysis of X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), temperature activation effects and activation time effects. The transesterification process analysis showed that the optimization conditions to produce biodiesel are 600 °C activation catalyst temperature, 90 minutes of catalyst-methanol mixing, 1.5% wt. catalyst concentration and 5 hours of transesterification time. The biodiesel yield was 96.9%. Moreover, new parameters were applied for this research (time and temperature of activation catalyst, catalyst-methanol mixing parameters and transesterification process conditions). Biodiesel properties (kinematic viscosity, flash point and water content) were measured according to ASTM D6751 standards and similarity was 98%. Therefore, biodiesel can be produced from canola oil and TiO2/CaO, but this still needs more studies on several topics such as the blending of canola with multi feedstocks, the ethanol impact and catalyst poisoning in the case of using TiO2-CaO as a catalyst.
Downloads