Theoretical Understanding of Quaternary Ammonium Surfactant Corrosion Inhibitor in Acetic Acid Media: Computer Modelling
DOI:
https://doi.org/10.37934/aram.114.1.112Keywords:
Corrosion Inhibitor, Quaternary ammonium, MD simulation, DFT calculationAbstract
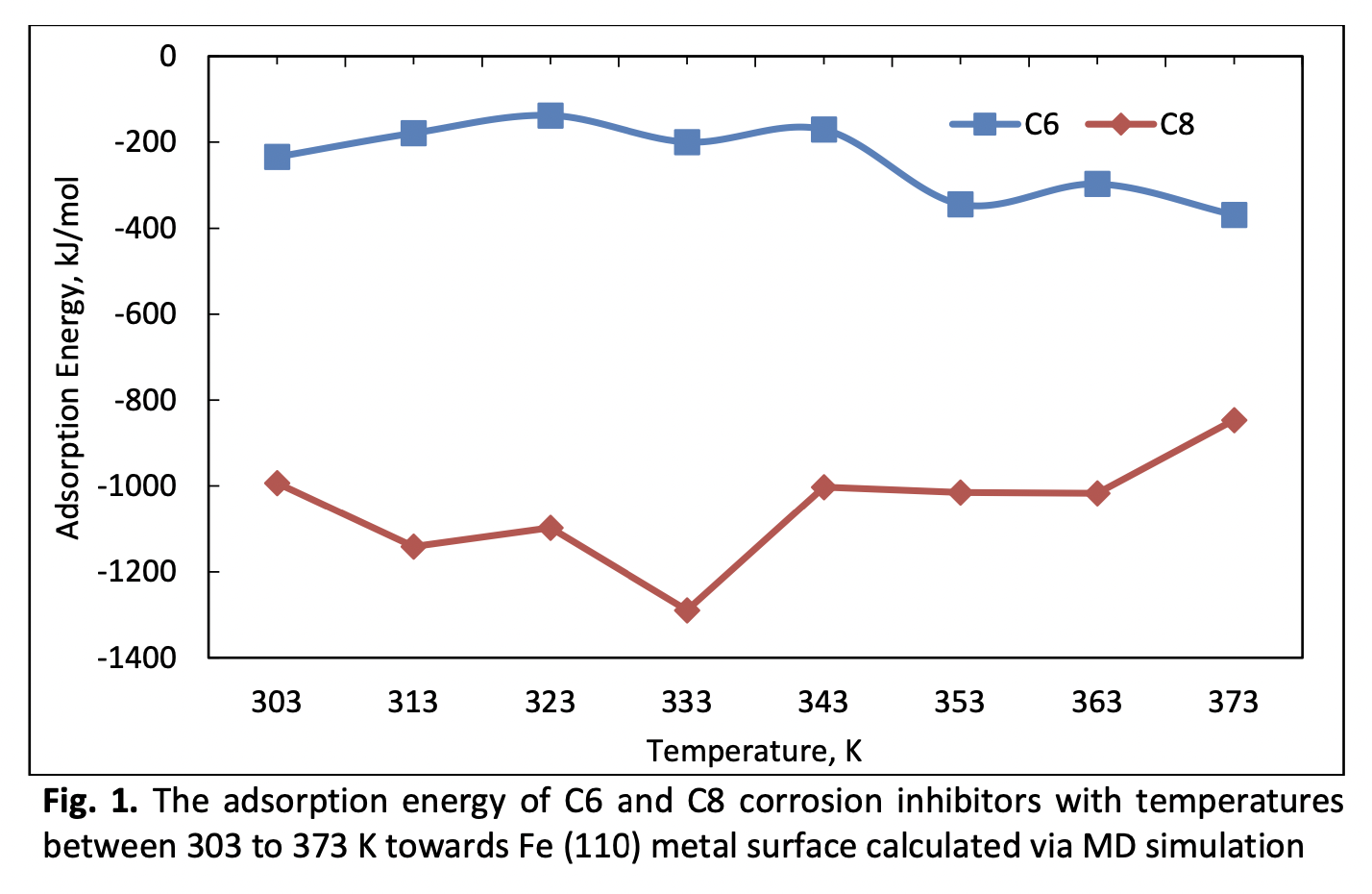
Corrosion inhibitor (CI) is one of the corrosion control strategies commonly used in controlling the rate of corrosion of metal equipment used in oil and gas production facilities. An in-depth analysis of the inhibition mechanism of CI molecules on the metal surface is crucial in understanding the corrosion theory. Hence, in this study, density functional theory (DFT) calculation and molecular dynamic (MD) simulation are used to investigate the inhibition mechanism of two quaternary ammonium cationic surfactant CI molecules, C6 and C8. Both CI molecules show a high reactivity in DFT calculation with a low band gap energy value (1.26 eV). The cationic ammonium of both CI molecules is the reactive region that donates electrons to the empty 3d orbital at a metal surface. Results from the MD simulation show that C8 CI molecules have a better inhibition property with a higher adsorption energy value. Other parameters, such as diffusion coefficient and molecular aggregation also used in explaining both CI properties as a corrosion inhibitor. The theoretical understanding analysis of the inhibition properties of CI molecules using computational methods can be used as a screening process for the future development of CI molecules that are more cost and time friendly.
Downloads